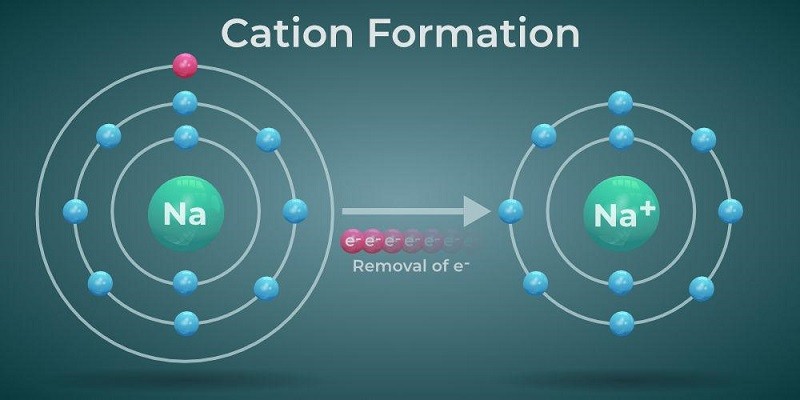
Cations are smaller than their parent atoms due to the loss of one or more outermost electrons. This loss of electrons reduces the electron-electron repulsion, resulting in a smaller size.
When atoms lose electrons to form cations, the number of protons remains the same but the number of electrons decreases, causing a stronger attraction between the remaining electrons and the nucleus. This increased attraction leads to a contraction of the electron cloud, making the cation smaller in size compared to the neutral atom.
The decrease in size is particularly noticeable in transition metals where the removal of electrons from the d-orbitals further strengthens the nucleus-electron attraction.
Why Cations Are Smaller: The Atomic Structure
Cations are smaller than their parent atoms due to the loss of electrons, resulting in a reduced electron cloud and a stronger positive charge on the remaining protons. This change in atomic structure causes the cations to have a smaller atomic radius.
The atomic structure plays a crucial role in understanding why cations are smaller than their parent atoms. Let’s delve into the details to uncover the fascinating reasons behind this phenomenon.
Structure Of An Atom In Brief:
- Atoms consist of three subatomic particles: Protons, neutrons, and electrons.
- Protons and neutrons are located in the nucleus, forming the central part of the atom.
- Electrons orbit the nucleus in energy levels or shells.
The Nature Of Cations:
Cations are positively charged ions formed when an atom loses one or more electrons. Here’s a closer look at the nature of cations:
- Cations have fewer electrons compared to their parent atoms due to the loss of one or more negatively charged electrons.
- The loss of electrons creates an imbalance in charge, resulting in a positive charge on the cation.
- Cations are typically formed by metals, as they readily donate electrons to achieve a stable electron configuration.
Relation To Atomic Size:
The reduction in size observed in cations can be attributed to several factors:
- Increased effective nuclear charge: With fewer electrons, the remaining electrons experience a stronger pull from the protons in the nucleus. This increased attraction results in a more compact electron cloud and ultimately leads to a smaller size.
- Decreased electron-electron repulsion: The fewer electrons in cations reduce the repulsion between electrons, allowing them to be pulled closer to the nucleus. As a result, the electron cloud becomes more compressed, contributing to a smaller atomic size.
- Loss of outermost energy levels: Cations often lose their outermost energy levels, which are typically larger and located farther from the nucleus. The removal of these larger energy levels results in a decrease in atomic size.
Understanding the atomic structure provides insights into why cations are smaller than their parent atoms. Factors such as increased effective nuclear charge, reduced electron-electron repulsion, and the loss of outermost energy levels all contribute to the reduced size of cations.
Electrons And Atomic Size
Electrons play a crucial role in determining atomic size. Cations, which are positively charged atoms, are smaller than their parent atoms due to the loss of one or more electrons, resulting in a stronger attraction between the remaining electrons and the nucleus.
Electron Orbitals: The Role They Play
When it comes to understanding why cations are smaller than their parent atoms, electron orbitals play a crucial role. Here’s why:
- Electrons in atoms occupy specific energy levels called orbitals.
- Each orbital has a maximum capacity for a certain number of electrons.
- Orbitals are grouped into different energy levels, including the s, p, d, and f orbitals.
- The s orbital is the lowest energy level and can hold a maximum of 2 electrons.
- The p orbitals have slightly higher energy levels than the s orbital and can hold a maximum of 6 electrons.
- The d and f orbitals have even higher energy levels and can accommodate more electrons.
How Valence Electrons Influence Atomic Size
Valence electrons, or the electrons in the outermost energy level of an atom, play a significant role in determining atomic size. Here’s how they influence it:
- Atoms with more valence electrons tend to be larger in size.
- This is because the outermost electrons experience less attraction from the positively charged nucleus, resulting in a larger atomic radius.
- As a general trend, moving across a period in the periodic table, the number of valence electrons increases, leading to a decrease in atomic size.
- Conversely, moving down a group, the number of energy levels increases, thus increasing the atomic size.
Electron orbitals and valence electrons have a substantial impact on why cations are smaller than their parent atoms. Understanding these factors helps explain the variations in atomic size across the periodic table.
Ionization And Atomic Size Reduction
Cations, the positively charged ions, are smaller than their parent atoms due to ionization and atomic size reduction. This occurs as electrons are lost, resulting in a decrease in electron-electron repulsion, leading to a smaller size.
The Process Of Ionization:
- When an atom loses one or more electrons, it forms a positively charged ion known as a cation.
- The process of ionization involves the removal of one or more electrons from the outermost shell of an atom.
- Electrons are negatively charged, and their removal creates an imbalance of positive and negative charges, resulting in a cation.
- Ionization can occur due to various factors such as exposure to high temperatures, high energy photons, or chemical reactions.
Effect Of Ionization On Atomic Size:
- Ionization leads to a reduction in the size of an atom, resulting in a cation being smaller than its parent atom.
- As electrons are removed during ionization, the outermost electron shell contracts.
- The attractive forces between the positively charged nucleus and remaining electrons pull the outer shell closer, causing a decrease in atomic radius.
- The decrease in atomic size is more pronounced for cations with multiple electron removals.
Comparing Cations And Neutral Atoms:
- Cations and neutral atoms differ in terms of size and electronic configuration.
- Cations are smaller than their parent atoms due to the loss of electrons and subsequent contraction of the outer electron shell.
- Compared to neutral atoms, cations have a higher effective nuclear charge, meaning the remaining electrons are more strongly attracted to the nucleus.
- The number of electrons in a cation’s outer shell is less than that in its parent atom, leading to different chemical properties and reactivity.
Ionization involves the removal of electrons from an atom, resulting in the formation of a cation. This process causes a decrease in atomic size as the outer electron shell contracts due to the diminished electron count. Cations differ from their parent atoms in terms of size and electronic configuration, with cations being smaller and exhibiting different chemical behaviors.

Credit: www.toppr.com
Frequently Asked Questions For Why Are Cations Smaller Than Their Parent Atoms?
Why Cations Are Smaller And Anions Are Larger Than Their Parent Atoms?
Cations are smaller and anions are larger than parent atoms due to electrons being gained or lost during the formation of ions.
Why Are Cations Always Smaller Than Anions?
Cations are smaller than anions because they lose electrons, resulting in a reduction in size.
Why Are Cations Smaller Than Their Parent Atoms?
Cations are smaller because they lose electrons, reducing the electron-electron repulsion and resulting in a smaller atomic radius.
What Causes Cations To Have A Smaller Size?
The loss of electrons in cations results in a decrease in the electron cloud, leading to a smaller size compared to the parent atoms.
Conclusion
The smaller size of cations compared to their parent atoms can be explained by the loss of one or more electrons during the ionization process. This loss results in a decrease in the electron-electron repulsion and a more pronounced pull from the nucleus, causing the remaining electrons to be pulled closer to the atom’s center.
As a result, the electron cloud becomes more compact, leading to a decrease in atomic radius. Additionally, the positively charged cations attract more electrons from their surrounding environment due to their electrostatic attraction, further shrinking their size. This phenomenon is crucial in understanding the behavior and properties of cations in various chemical reactions and how they interact with other atoms and molecules.
By comprehending the underlying principles behind the size difference, scientists can better design and predict the behavior of cations in different chemical systems, contributing to diverse areas such as material science, catalysis, and biochemistry.